
In June, Applied Policy’s diagnostic experts introduced a new series, “IVD Test Reimbursement: Coverage, Coding, and Payment.” For our July edition, we take a deeper dive into the first reimbursement consideration: coverage.
What is coverage?
Coverage refers to whether an insurer will pay for a particular item or service. In general, Medicare and most private payers limit coverage to clinically proven lab tests that are reasonable and necessary for the diagnosis or treatment of an illness or injury (and within the scope of a Medicare benefit category). For example, Medicare Part B covers a Human Immunodeficiency Virus (HIV) screening once per year if the patient is age 15-65. Coverage is also extended to patients younger than 15 and older than 65, who are at increased risk for HIV, and pregnant women can be screened up to three times during pregnancy.[1] Alternatively, coverage may be denied, as seen in Cardiovascular (CV) Risk Screening, NCD 190.23. This policy denies coverage for all CV risk assessment panels, except the basic lipid panel, for symptomatic patients with suspected or documented CV disease because panel testing is not specific to a given patient’s lipid abnormality or disease.[2]
Medicare provides coverage for items and services for over 62 million beneficiaries.[3] Most of the coverage is provided on a local level, developed by Medicare Administrative Contractors (MACs). A MAC is a private health care insurer that has been awarded a geographic jurisdiction to process Medicare claims for Medicare Fee-For-Service (FFS) beneficiaries.[4] The Centers for Medicare & Medicaid Services (CMS) relies on a network of MACs to serve as the primary operational contact between the Medicare FFS program and the health care providers enrolled in the program.[5]
In certain cases, Medicare deems it appropriate to develop a nationwide policy or a National Coverage Determination for an item or service for all Medicare beneficiaries meeting the criteria for coverage. Let’s look at both types of these coverage policies.
National Coverage Determinations (NCDs)
NCDs are nationwide determinations of whether Medicare will pay for an item or service.[6] NCDs are made through an evidence-based process, with opportunities for public participation. In some cases, CMS’s own research is supplemented by an outside technology assessment and/or consultation with the Medicare Evidence Development & Coverage Advisory Committee (MedCAC).[7] The MEDCAC is used to supplement CMS’ internal expertise and to allow an unbiased and current deliberation of “state of the art” technology and science. The MEDCAC reviews and evaluates medical literature, reviews technology assessments, public testimony and examines data and information on the benefits, harms, and appropriateness of medical items and services that are covered under Medicare or that may be eligible for coverage under Medicare. The MEDCAC judges the strength of the available evidence and makes recommendations to CMS based on that evidence.
NCDs are relatively rare, making up approximately 10 percent of all coverage policies. An NCD exists for thirty-three of the most common clinical laboratory tests, including tests for fecal occult blood, blood glucose, lipids, prothrombin time, prostate specific antigen, and HIV Viral Load.[8]
As part of the NCD process, CMS may cover an item or service for Medicare beneficiaries on the condition that they are furnished in a setting of ongoing data collection (i.e., in the context of a clinical study). This process called coverage with evidence development (CED).[9] For example, pharmacogenomic testing for warfarin response is covered under CED.[10]
Given that evidence supporting coverage may develop over time, NCDs may undergo revisions based on reconsiderations. For example, in February 2022, NCD 210.14, Screening for Lung Cancer with Low Dose Computed Tomography (LDCT), was revised to expand beneficiary eligibility by lowering the minimum age for screening to 50 years.[11]
Local Coverage Determination (LCD)
LCDs are more common than an NCD. LCDs are decisions made by a MAC to apply or deny Medicare coverage within that MAC’s jurisdiction.[12] LCDs can develop on a topic not covered by a NCD or on a point on which the NCDs are silent, but LCDs cannot conflict with NCDs.
LCDs may also be reconsidered and subsequently revised. For example, in June 2022, based on recommendations from the College of American Pathologists, the American Society of Clinical Oncologists (ASCO), and the National Comprehensive Cancer Network (NCCN), Palmetto GBA determined that hormone receptor assays, estrogen receptor (ER), progesterone receptor (PR), and Her-2/neu by immunohistochemistry (IHC) is covered for patients with primary invasive breast cancers and recurrent or metastatic cancers.[13]
Coverage for new IVD tests
One of the major challenges facing diagnostic test manufacturers is convincing Medicare and private health insurers to pay for new tests. This issue has become even more urgent as IVD tests become more complex and the healthcare industry focuses on improving quality of care and reducing costs. Providing persuasive, data-driven evidence showing that paying for a new test or service will improve patient outcomes and reduce healthcare costs is imperative.[14]
Clinical Utility
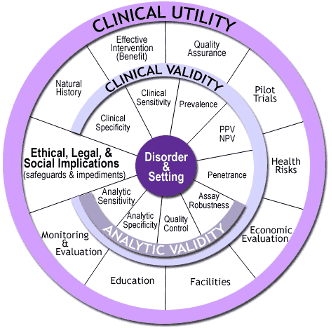
To secure Medicare and private payer reimbursement for a new test or service, rigorous, data-driven evidence is required. As new genomic IVD tests began to emerge, the Center for Disease Control’s (CDC’s) Office of Public Health Genomics (OPHG) established and supported the ACCE Model Project, which developed the first publicly-available analytical process for evaluating scientific data on emerging genetic tests.[15] This model process includes collecting, evaluating, interpreting, and reporting data about DNA (and related) testing for disorders with a genetic component in a format that allows policy makers to have access to up-to-date and reliable information for decision making. It serves as a comprehensive blueprint for manufacturers to use as they develop new tests.
ACCE stands for the four main criteria used by payers for evaluating a genetic test — analytic validity, clinical validity, clinical utility and associated ethical, legal, and social implications. Specifically, the ACCE model process is composed of a standard set of 44 targeted questions [16] that address disorder, testing, and clinical scenarios, as well as analytic and clinical validity, clinical utility, and associated ethical, legal, and social issues.
Ultimately, evidence of clinical utility – the ability of the new test to alter the way patients are managed and/or improve net health outcomes – determines Medicare and private payer coverage decisions. While payers may have varying definitions of clinical utility all payers are increasingly requiring evidence of improved health outcomes as a condition for coverage.
Stay tuned for next month’s Applied Policy Insights in which our diagnostics team examines the second component of IVD reimbursement: coding.
[1] https://www.medicare.gov/coverage/hiv-screenings
[2] https://www.cms.gov/medicare-coverage-database/view/lcd.aspx?LCDId=36358
[3] https://www.statista.com/topics/1167/medicare/
[4] Fee-for-service is a system of health care payment in which a provider is paid separately for each particular service rendered. Original Medicare is an example of fee-for-service coverage, and there are Medicare Advantage plans that also operate on a fee-for-service basis. https://www.healthcare.gov/glossary/fee-for-service/
[5] https://www.cms.gov/Medicare/Medicare-Contracting/Medicare-Administrative-Contractors/What-is-a-MAC#:~:text=A%20Medicare%20Administrative%20Contractor%20(MAC,%2DService%20(FFS)%20beneficiaries.
[6] https://www.cms.gov/Medicare/Coverage/DeterminationProcess
[7] https://www.cms.gov/Regulations-and-Guidance/Guidance/FACA/MEDCAC
[8] https://www.cms.gov/medicare-coverage-database/reports/national-coverage-ncd-report.aspx?chapter=190&sortBy=chapter
[9] https://www.cms.gov/Medicare/Coverage/Coverage-with-Evidence-Development
[10] https://www.cms.gov/Medicare/Coverage/Coverage-with-Evidence-Development/Pharmacogenomic-Testing-for-Warfarin-Response
[11] https://www.cms.gov/medicare-coverage-database/view/ncd.aspx?NCDId=364
[12] https://www.cms.gov/Medicare/Coverage/DeterminationProcess/LCDs
[13] https://www.cms.gov/medicare-coverage-database/view/article.aspx?articleid=53704&ver=22&contractorName=all&contractorNumber=all&updatePeriod=996&sortBy=updated&bc=13
[14] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6787613/
[15] https://www.cdc.gov/genomics/gtesting/acce/index.htm
[16] https://www.cdc.gov/genomics/gtesting/acce/acce_proj.htm
