
On September 23, 2024, the Centers for Medicare & Medicaid Services (CMS) held an education session regarding the August 7, 2024 final procedural notice, which finalized the Transitional Coverage for Emerging Technologies (TCET) pathway (CMS-3421). On August 7, CMS also published final guidance documents on Coverage with Evidence Development, National Coverage Analysis Evidence Review, and Clinical Endpoints Guidance for Knee Osteoarthritis. While this session focused on sharing information and answering questions on these guidance documents and the TCET pathway established in the final notice, CMS anticipates proposing a fit-for-purpose study design and a real-world data study protocol guidance soon. CMS states that a prioritization guidance will also be published after they have worked through several quarterly review cycles of TCET nominations. Some of the slides shared during the education session are included in this summary.
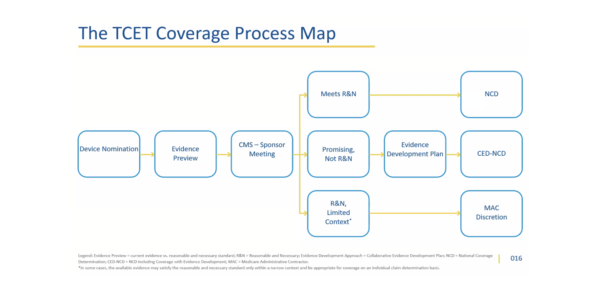
Overview of TCET Pathway
The Center for Clinical Standards and Quality (CCSQ) presented key takeaways from the TCET final notice and future directions CMS has planned to implement this pathway. CMS believes TCET will improve the national coverage determination (NCD) process for stakeholders through pre-market, near-market, early post-market, and post-market engagement stages.

CMS also noted that a proposed fit-for-purpose study design and real-world data study protocol guidance will be published soon. Based on stakeholder feedback and data collected, CMS indicated that these types of studies more accurately reflect current clinical settings and allow for greater flexibility of study design. In discussion of the agency’s long-term plans, CMS indicated they hope to release prioritization guidance after several quarterly review cycles to increase TCET pathway transparency.

While concerns were raised that the proposed TCET notice took over a year to finalize, CMS used this period to pilot test concepts in the TCET pathway to ensure they would work for all manufacturers. CMS also used this time to engage with partner agencies and identify opportunities for improved coordination.
Furthermore, CMS aims to finalize TCET NCDs as early as six months after FDA market authorization, provided there is sufficient early engagement and timely device collaboration, and so long as the coverage group is adequately resourced. CMS noted the coverage with evidence development requirements would continue only as long as necessary to facilitate evidence generation that can inform patient and clinician decision-making and lead to predictable, long-term Medicare coverage.
CMS stated stakeholder feedback will continue to be incorporated throughout the TCET pathway process. To be considered for the first quarterly review cycle, device nominations must be submitted by October 31st, 2024.
Questions and Answers
CMS staff answered pre-selected questions at the end of the session. Live questions were not accepted. Steve Farmer, Chief Strategy Officer and Deputy Group Director, and Lori Ashby, Senior Advisor at the CMS Coverage Analysis Group, provided some insightful responses, as underlined below.
Question: When does the one-year clock start for nominations? Additionally, what happens if there are delays in FDA market authorization?
- Answer: Under the TCET pathway, CMS will conduct extensive work in the pre-market period to shorten coverage review time frames after devices are FDA market authorized. As we stated in the final notice, we believe 12 months before the anticipated FDA market authorization is the appropriate time frame for TCET procedural steps to be completed and for better coordination of coding and payment. This timeframe is not tied to the quarterly review cycle or the date the final notice was released. The final notice includes an opportunity for a manufacturer to submit a nonbinding letter of intent to nominate a potentially eligible device approximately 18 to 24 months before the manufacturer anticipates FDA marketing authorization. While formal nominations will still be considered approximately 12 months before anticipated market authorization, the submission of a nonbinding letter of intent will improve CMS’s ability to track potential candidates, coordinate with FDA, and make operational adjustments. Nominated devices will be assessed against eligibility criteria and then prioritized every quarter. We recognize that market authorization dates may change for various reasons. Delays in FDA market authorization would not impact acceptance into the pathway, though FDA market authorization is needed for coverage.
Question: Is there a lookback period? More specifically, are Breakthrough Devices nearing an FDA decision on market authorization (less than 12 months), or those recently achieving FDA market authorization, eligible for TCET?
- Answer: We did not include a lookback period in the proposed or final notice. Devices already on the market are not appropriate for the TCET pathway. TCET is designed to accelerate the NCD process in the post-market period by initiating reviews in the pre-market phase. Developing an Evidence Development Plan (EDP) generally takes considerable time. Devices already available on the market, or those already close to FDA market authorization, are more appropriate for an NCD outside the TCET pathway or coverage at the local level through a local coverage determination (LCD) or claim-by-claim adjudication.
Question: Can CMS describe how software as a medical device and/or other digital health innovations fit into the TCET pathway?
- Answer: To the extent that these technologies meet the criteria described in the final procedural notice, appropriate candidates may be eligible. However, we know that any technology seeking Medicare coverage is required by statute to fall within a Medicare benefit category under Part A or Part B. Establishing one or more benefit categories for software as a medical device technology is an area of active exploration and policy development within CMS. There is also interest at the Congressional level as well.
Question: In the TCET final notice, CMS generally excludes diagnostic lab tests, citing that diagnostic lab tests are considered a highly specific area of coverage policy development. However, diagnostic lab tests are regulated as medical devices and equally eligible for FDA Breakthrough Designation. What is it about diagnostics that is different from other medical devices that excludes them from the TCET pathway?
- Answer: While the TCET pathway is open to FDA Breakthrough Devices, CMS expects that the majority of coverage determinations for Breakthrough Designated diagnostic laboratory tests will continue to be made by the MACs. We acknowledge that there may be instances where manufacturers and CMS agree that an NCD is appropriate for a diagnostic laboratory test. In those instances where manufacturers believe that additional evidence generation may be needed to satisfy the Medicare coverage standard, we encourage manufacturers to contact CMS to discuss options for their specific technology.
Question: Can you elaborate on how CMS will prioritize TCET nominations? How is beneficiary impact defined? Will CMS consider the impact of Breakthrough Designated device treatment on Medicare beneficiaries suffering from high costs and/or less common diseases for which limited, or no treatment options exist? Will CMS look at conditions that impact the greatest number of beneficiaries regarding disease prevalence or employ some other criteria? And finally, will CMS consider health equity impact?
- Answer: Until we release more specific prioritization factors, CMS will prioritize eligible devices based on the 2013 Federal Register notice. This 2013 notice states that in the event we have a large volume of NCD requests for simultaneous review, we will prioritize these requests based on the magnitude of the potential impact on the Medicare program and its beneficiaries and staffing resources. A high impact on the Medicare program may be assessed based on a significant benefit for a relatively small number of patients, or a modest benefit for a relatively large number of patients. The current administration has engaged in extensive efforts to address health disparities through numerous initiatives. All things being equal, we will consider whether a device may have a health equity impact.
Question: How will CMS prioritize nominations if qualified nominations are moved from one quarterly cycle to the next? Will these devices be re-prioritized against new nominations in the subsequent cycle? When re-evaluating a device that was moved to the next review cycle, will priority be given to a device that may soon be outside of the optimal window for the pathway? Specifically, devices within six months of anticipated FDA market authorization?
- Answer: CMS will prioritize TCET devices within each quarterly review cycle. If not accepted in the initial quarterly review cycle, it will be automatically reconsidered in the subsequent cycle. Manufacturers do not need to resubmit their nominations. Within each quarterly review cycle, devices will be evaluated on their individual merits. Since TCET is forward-looking and extensive pre-market engagement is essential, nominations for Breakthrough Devices anticipated to receive an FDA decision on market authorization within 6 months may not be accepted because CMS would be unable to reach a final NCD within the expedited time frames. A nominated device that is not accepted in the first review may be accepted during a subsequent review even though FDA’s decision on market authorization is anticipated within six months. If this occurs, CMS will work with the manufacturer to expedite the review as practically achievable. If devices are approved with a shorter than ideal pre-market review period, an NCD may be delayed post-market.
Question: What level of detail will CMS provide to manufacturers whose nominations are declined for reasons other than the cap being met?
- Answer: If we decline a nomination, CMS will provide justification and contact information for additional information. We will identify why the nomination has been declined, including the absence of an FDA Breakthrough Designation, a benefit category determination, the device being subject to an existing Medicare NCD, or it being excluded from coverage through law or regulation.
Question: The TCET final notice states that candidates not selected for TCET in a quarterly review cycle will be automatically considered in the next cycle. Will CMS notify applicants if they are no longer automatically considered in the next cycle (due to proximity to anticipated FDA approval)?
- Answer: CMS is developing a web-based system that will automatically notify manufacturers of any status updates for TCET nominations, including whether they are eligible, have been accepted into the pathway, will be automatically reconsidered in a subsequent quarter, or will no longer be considered for the pathway. We expect the system to be operational by the end of 2024. If devices are approved with a shorter than ideal pre-market review period, NCD may be delayed post market.
Question: How and when will CMS make the following information public after the close of each nomination cycle? First is the number of TCET applications CMS received and the second is the number of a device procedure class of candidates accepted into the TCET program. And how long after the close of the nomination cycle will CMS update the NCD dashboard with the number of candidates and device procedure classes accepted into the program? What level of detail will CMS share regarding how candidates were selected and what details will be given to submitters?
- Answer: TCET nominations are voluntary and confidential, and CMS cannot publish a nomination list. CMS will include information such as the number of devices in the TCET pathway, the date of nomination, the date of acceptance, and the date the NCD process is initiated. We intend to update the NCD dashboard quarterly.
Question: How does CMS use the TCET nomination material to inform the Evidence Preview (EP)? Will the list of studies in the TCET nomination serve as the basis for the EP? Or will a new literature search be conducted as part of the EP?
- Answer: In piloting the EP concept, we found that terminology changes occurred for some emerging technologies during development. This resulted in some publications being omitted from our systematic literature review that were relevant to the EP. To avoid revisions to the document, and the delays that may subsequently occur, we request that manufacturers list all potentially relevant literature in their nomination request. To ensure completeness and avoid revisions, the contractor will conduct a systematic literature review and compare it against the manufacturer’s documents by bibliography. If a critical article was not included in the systematic literature review, it will be added into the review. I would also like to highlight the collaborative nature of the evidence review process. With contractor support, CMS develops the draft EP and then shares it with the Agency for Healthcare Research and Quality (AHRQ) for feedback. The manufacturer then has an opportunity to propose technical edits and corrections to the document and may add substantial additional language in the appendix if needed. The intent is to establish a shared understanding of the state of the evidence that can inform a stakeholder meeting about the best available coverage options.
Question: According to the final notice, manufacturers can voluntarily submit letters of intent 18 to 24 months before anticipated authorization. Can CMS provide insight on the purpose of letters of intent and describe how submitting a letter of intent could benefit the manufacturer?
- Answer: The voluntary letter of intent aims to provide CMS with greater predictability regarding the approximate timing and nature of potential TCET nominations. Advance notice will help CMS to optimize the pathway. Specifically, the submission of a nonbinding letter of intent will improve CMS’s ability to track potential candidates, coordinate with FDA, and make operational adjustments. Additionally, a nonbinding letter of intent can help alleviate potential delays of a clinical endpoints review. Regardless of whether manufacturers submit a letter of intent, they are encouraged to nominate the device approximately 12 months before anticipated FDA market authorization to make optimal use of the pre-market review time.
Question: Is CMS planning any best practice training on how to submit a nomination?
- Answer: Yes. We plan to conduct a workshop to assist manufacturers with the submission process. We will have additional details soon.
Question: The final TCET notice recommends that nominations be submitted approximately 12 months before the manufacturer anticipates an FDA decision on market authorization. This suggests heavy reliance on a process governed by another agency. How will CMS and FDA coordinate on the TCET process?
- Answer: Over the last year, CMS staff members have regularly met with FDA, AHRQ, manufacturers, and others to improve coordination across the government and with innovators. CMS staff are also regularly engaging with the FDA total lifecycle product advisory program. Additionally, as we stated in the final notice, representatives from CMS may meet with FDA to learn more information about specific technologies after CMS initiates a review of a complete formal nomination. These discussions will help CMS better understand the device and the timing of potential FDA reviews. Initiation of the TCET process approximately one year before anticipated FDA market authorization is intended to be close enough to FDA market authorization that the EP can incorporate pivotal trial results. If there are material evidence gaps, the manufacturer will have sufficient time to develop an EDP.
Question: How will CMS address coding and payment for devices accepted into TCET?
- Answer: TCET allows CMS to better align coding and payment processes with existing review time frames by initiating a review well before FDA market authorization. CMS encourages manufacturers to proactively pursue codes and not delay submitting TCET nominations. To help manufacturers navigate the process, CMS has established a Pharmaceutical and Technology Ombudsman to help coordinate coverage, coding, and payment and has published an online guide. The guide will soon be updated to include information on TCET.
Question: For a manufacturer of a follow-on device, how much time before FDA approval can the EP and EDP be initiated? Should manufacturers contact CMS to initiate an EP if a TCET candidate for a similar device has been accepted and an NCD is underway?
- Answer: If an applicable NCD with Coverage Evidence Development (CED) requirements has been opened or is anticipated, second-to-market devices are encouraged to engage with CMS approximately 12 months before anticipated FDA market authorization. This is so they can initiate an EP, specific to the device, and so the manufacturer has sufficient time to develop an EDP. If CMS is aware of a second-to-market device, CMS may also proactively engage with the manufacturer to initiate both of those processes. Delays in developing an EP or EDP may delay the establishment of an NCD after the device is on the market.
Question: If a manufacturer-sponsored CED study is required for coverage for a follow-on technology, is there noncoverage for a follow-on device until there is a CMS approved CED study? And how can this be minimized?
- Answer: Under the Social Security Act, CMS will nationally cover an item or service only in the context of an approved clinical study or with the collection of additional clinical data. The second-to-market device will be noncovered until a device specific EDP and CED study is approved. This delay could be avoided entirely by initiating the EP as soon as possible if there is an expected or established CED NCD.
Question: How will CMS prioritize the EP and EDP review of follow-on devices? Has CMS accounted for this in its TCET resource allocation?
- Answer: We believe CMS has sufficient resources to conduct timely EPs and work with manufacturers on EDPs for follow-on devices, as well as those within the pathway.
Question: We understand that CMS now utilizes a contractor to help with the technical analysis. Has this decreased the processing time for new NCDs and reconsideration requests? How will CMS accomplish the TCET workload in addition to the typical number of non-TCET NCDs?
- Answer: With the addition of TCET, CMS has more than doubled our annual NCD volume without additional federal full-time employees. CMS has leveraged operational efficiencies to streamline and standardize the evidence review process wherever possible. We have augmented our available resources with contractor support, often allowing us to incorporate specialized clinical expertise into the review. These operational improvements will apply to all of our reviews and finding efficiencies across all of our work was necessary in order to add to the additional workload of TCET. Additionally, we note some potential overlaps as some Breakthrough Devices would have also featured on the NCD waitlist if they weren’t accepted into the TCET pathway.
Question: What is the anticipated timing of future CMS guidance? Such as fit-for-purpose (FFP), real-world data study protocol, and prioritization guidance?
- Answer: We anticipate releasing FFP study and real-world data study protocol guidance soon. We anticipate publishing the proposed prioritization guidance after we have worked through several quarterly review cycles.
Question: CMS states that technologies within six months of FDA market authorization will not be accepted into the TCET pathway. How will CMS prioritize coverage for these technologies under the traditional coverage pathway?
- Answer: The manufacturer of a Breakthrough Device not accepted into the TCET pathway may submit a complete formal NCD request if they wish to pursue a conventional NCD. The 2013 federal register notice states that in the event we have a large volume of NCD requests for simultaneous review, we prioritize these requests based on the magnitude of the potential impact on the Medicare program and its beneficiaries and staffing resources.
Question: If Medicare Advantage plans must cover all medically necessary services that original Medicare covers, would that include devices under TCET?
- Answer: Yes. Medicare Advantage plans must comply with TCET NCDs just as they do with conventional NCDs.
Download a copy of this summary here.
